
Key Takeaways
- Policy adherence is no longer about documentation but interpretation. Modern pharmaceutical compliance depends on understanding and reasoning through frequent regulatory and procedural changes, not just following static checklists.
- Traditional automation can’t keep up with evolving rules. RPA and static compliance tools fail when the intent or meaning of a regulation changes, creating hidden gaps between policy and execution.
- Agentic systems bring reasoning into compliance. These AI-driven frameworks can read, interpret, and apply policy updates—linking them to affected SOPs, training records, and validation processes in real time.
- Human expertise remains central. Agentic adherence doesn’t replace QA professionals; it augments their oversight, freeing them to focus on contextual judgment and strategic decision-making.
- The future of compliance is adaptive and trust-driven. As organizations begin to trust machine reasoning for traceable, explainable policy interpretation, agentic systems will become foundational to pharma QA operations.
There’s a quiet truth in pharmaceutical manufacturing that rarely makes it into glossy annual reports: compliance isn’t about paperwork anymore. It’s about interpretation. The number of policies, frameworks, and revisions that a single plant has to juggle today is staggering. Every year brings a new wave—updated GMPs, local regulatory nuances, internal quality revisions, and supplier agreements.
If you walk into a formulation unit, you’ll see it right away. Operators and supervisors are technically following procedures, but the speed at which those procedures evolve leaves everyone slightly uncertain. One department updates its SOPs, another hasn’t caught up yet, and QA is left piecing together the aftermath. The irony? Most deviations don’t happen because people ignore policies—they happen because the system that’s supposed to enforce them can’t keep up.
Also read: Agentic AI: The Future of Autonomous Decision-Making in Enterprises
When Rules Move Faster Than People
Policies age faster than products. That’s the uncomfortable reality. A change in equipment qualification requirements from the FDA can ripple through cleaning protocols, validation timelines, and vendor audit schedules. But that change doesn’t instantly reach the shop floor.
You can automate forms, track training logs, or run audits with RPA bots—but those systems still rely on static logic. They can tell you if a field is missing or if a document isn’t signed. What they can’t tell you is that the meaning behind a rule has shifted.
For instance, suppose a manufacturer switches to a new disinfectant because the old one was discontinued. The relevant cleaning validation SOP might have changed to reflect new residue testing requirements. The RPA bot still checks that “a test result exists,” but it has no sense of whether that result aligns with the latest rule set.
That’s the heart of the problem: policy adherence is not a checklist; it’s an ongoing act of reasoning.
The Shift Toward Agentic Policy Systems
Over the last two years, a handful of advanced pharma companies have started experimenting with agentic systems—AI frameworks that behave less like tools and more like reasoning entities. The difference is subtle but profound.
Where traditional automation executes commands, agentic systems interpret instructions. They can read a policy update, understand what processes it affects, and trigger the necessary changes downstream.
Imagine a digital compliance analyst who never sleeps. When a new regulatory bulletin lands from the EMA, it parses the text, identifies which sections align with your current SOPs, and maps the potential impact. Then it notifies QA, updates internal documentation links, and flags the training modules that need revision.
No human could do this at the same pace—not without missing something.
The Anatomy of Agentic Policy Adherence
To visualize how agentic policy adherence transforms compliance workflows, it helps to break down the process into four interconnected layers:
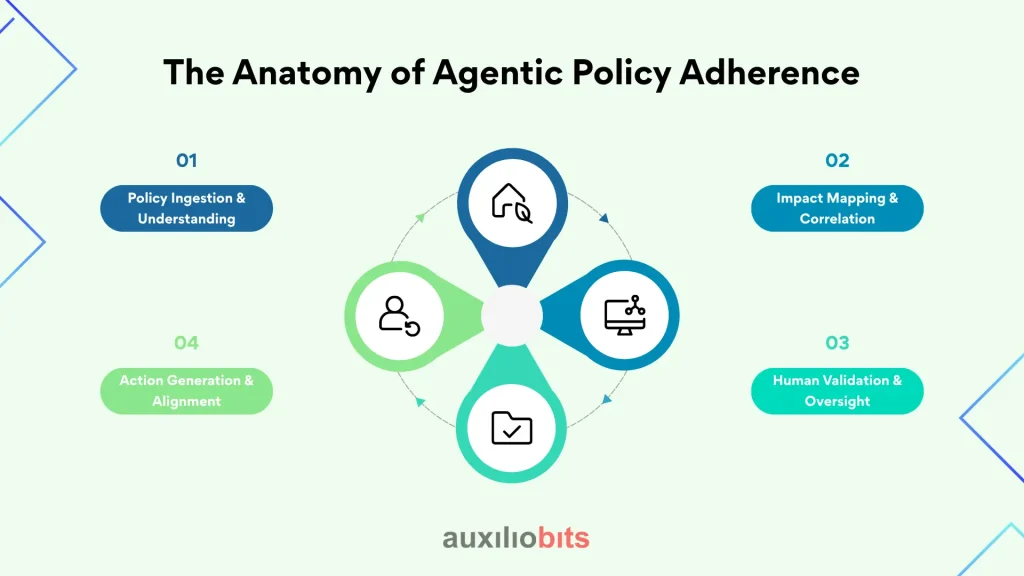
- Policy Ingestion & Understanding: Agents continuously scan new regulations, GMP updates, and internal policy changes. They interpret the intent behind each clause—not just keywords or checklists.
- Impact Mapping & Correlation: The system identifies where the change applies—linking it to affected SOPs, validation documents, supplier audits, and training modules. This creates a living dependency graph of compliance elements.
- Action Generation & Alignment: Instead of just issuing alerts, the agent generates actionable items—revision drafts, retraining tasks, and CAPA linkages—automatically aligning them with regulatory expectations.
- Human Validation & Oversight: QA professionals review, approve, or fine-tune the AI’s recommendations, ensuring contextual accuracy and ethical compliance before final implementation.
This structure makes it clear how agentic systems elevate compliance from reactive monitoring to proactive reasoning—a perfect foundation for a visual infographic showing the end-to-end flow.
Why Static Compliance Fails in Dynamic Environments
In real-world pharma operations, the messiness of human systems collides with the rigidity of digital ones. A manufacturing line could be running three concurrent campaigns with slightly different specifications, each tied to a different regulatory region. Policies overlap, contradict, or become outdated mid-cycle.
When everything is static, you get confused. When everything is adaptive, you risk chaos. The middle ground is agentic reasoning—systems that understand the intent behind compliance language and can apply it appropriately based on context.
For example:
- A temperature excursion in a stability chamber triggers a CAPA. The agent checks which product batches are affected, refers to the current ICH Q1A guidelines, and advises whether data can still be considered valid or if retesting is mandatory.
- A policy update redefines “critical deviation.” The agent automatically recalibrates risk classifications in the deviation tracking system and highlights records that now fall under the new definition.
- During supplier qualification, the agent reads audit reports and determines whether new findings breach existing quality agreements.
These are not hypothetical capabilities anymore—they’re slowly surfacing in pilot programs across large manufacturing networks.
What “Policy Adherence” Really Means in Practice
There’s a misconception that adherence equals enforcement. In truth, adherence in pharma means alignment—between intent, action, and documentation. You can follow every SOP step correctly and still fail an audit if the SOP itself is outdated.
Agentic systems approach this differently. They maintain a living map of every rule, document, and related process. So when a clause changes in one place, its ripple effects are instantly visible.
Let’s take training as a simple example. When a batch record form changes, QA updates the SOP, and the system automatically checks who has been trained on the previous version. It then schedules retraining for the affected roles without anyone raising a ticket.
That might sound trivial, but multiply it by hundreds of policies, dozens of production lines, and multiple global markets—and suddenly, it’s the difference between a compliant organization and one skating on the edge of warning letters.
When Automation Becomes Interpretation
We’ve spent a decade chasing “automation maturity,” but the truth is, automation without comprehension hits a ceiling fast. You can automate document routing or signature collection all day long—it won’t help when the underlying rulebook shifts.
Agentic adherence bridges that gap. These systems:
- Read policies like humans—parsing language, extracting intent, not just keywords.
- Correlate dependencies—knowing which systems, templates, or validations are touched by a single rule change.
- Generate contextual actions—not just alerts, but specific, traceable tasks tied to regulatory expectations.
This turns compliance from a reactive activity into a continuous, adaptive process.
Real-World Example: A Contract Manufacturer’s Experience
A mid-sized contract manufacturing organization (CMO) faced recurring issues during customer audits. The auditors kept finding small inconsistencies—training matrices not reflecting updated job roles, outdated risk assessments linked to obsolete SOPs, that kind of thing. Nothing critical, but enough to raise eyebrows.
When they implemented an agentic policy layer—essentially a set of AI-driven compliance agents connected to their document control and LIMS systems—the effect was immediate.
The system began flagging documents that referenced deprecated clauses. It identified missing CAPA linkages that humans had overlooked. During a mock audit, QA leads noticed that policy deviations were being detected days earlier than before, sometimes even before a batch was released.
The plant manager described it best: “It’s like having a second set of eyes that never gets tired but also understands why something matters.”
The Human Side of Intelligent Compliance
Of course, there’s an unspoken concern. Every time you bring intelligence into compliance, people worry about being replaced. But policy adherence is one of those domains where human judgment is irreplaceable.
Agents don’t replace QA professionals—they amplify them. They handle the cognitive overload, leaving people to focus on interpretation, ethical judgment, and strategic oversight.
One could argue that the more these systems understand policy intent, the more they’ll rely on human context. Machines can detect a deviation, but only a person can decide whether it reflects a systemic weakness or a one-time oversight.
The Road Ahead
Agentic policy adherence is still a young concept. Most systems are in proof-of-concept or pilot stages. But the trajectory is unmistakable. As policies evolve faster and the complexity of manufacturing networks deepens, static compliance tools will simply fall behind.
The challenge won’t be adoption—it’ll be trust.
Can organizations trust machine reasoning in something as unforgiving as pharma compliance? Probably not at first. But over time, as these systems show traceability and consistency, they’ll become part of the normal QA fabric.
It’s the same way people once resisted digital batch records and now can’t imagine operating without them. The leap from data automation to policy reasoning will follow the same arc—quietly, inevitably.
Final Thought
Pharmaceutical compliance has always been about the dance between precision and interpretation. Agentic systems don’t replace that dance; they make the rhythm more manageable. Instead of policing every move, they keep the beat steady—so humans can focus on the steps that truly matter








